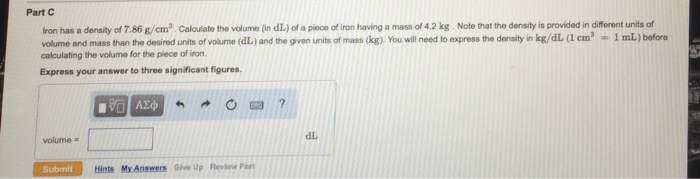
Iron has a density of 7.86 g/cm3 – Iron, with its density of 7.86 g/cm3, stands as a material of immense significance in the realm of science and engineering. Its unique properties, largely influenced by its density, have shaped its diverse applications across various industries.
Delving into the concept of density, we define it as the mass of a substance per unit volume. Measured in grams per cubic centimeter (g/cm³), density provides insights into the compactness and arrangement of atoms within a material.
Iron’s Density: Iron Has A Density Of 7.86 G/cm3
Iron’s density is a physical property that measures the mass of iron per unit volume. It is an important property that affects the behavior and applications of iron in various fields.
Definition and Units of Density
Density is a measure of how tightly packed the particles of a substance are. It is defined as the mass of a substance per unit volume. The most common unit of density is grams per cubic centimeter (g/cm³). This means that the density of a substance is the number of grams of that substance that would fit into a cube with sides that are each 1 centimeter long.
Density can also be expressed in other units, such as kilograms per cubic meter (kg/m³). However, g/cm³ is the most commonly used unit for density in scientific and engineering applications.
Iron’s Density: Iron Has A Density Of 7.86 G/cm3
Iron, a prominent element, possesses a density of 7.86 g/cm³, a value that significantly influences its characteristics and diverse applications.
Value and Significance, Iron has a density of 7.86 g/cm3
The density of iron, 7.86 g/cm³, represents the mass of iron per unit volume. This value is crucial for understanding the material’s properties, such as its strength, durability, and thermal conductivity.
Comparison with Other Materials

Iron’s density is a crucial property that sets it apart from other materials. To provide a comprehensive perspective, we present a comparative analysis of iron’s density with several common materials.
The following table summarizes the density values and brief descriptions of iron and other selected materials:
Table: Density Comparison
| Material | Density (g/cm3) | Description |
|---|---|---|
| Iron | 7.86 | A strong, ductile, and magnetic metal |
| Aluminum | 2.70 | A lightweight, corrosion-resistant metal |
| Copper | 8.96 | A highly conductive and malleable metal |
| Water | 1.00 | A colorless, odorless, and tasteless liquid |
As evident from the table, iron exhibits a significantly higher density compared to aluminum and water. This difference in density is attributed to the varying atomic masses and packing arrangements of the respective materials.
Iron’s high density makes it suitable for applications requiring strength, durability, and weight-bearing capacity. Conversely, aluminum’s low density makes it ideal for applications where lightweight and corrosion resistance are critical.
Factors Affecting Iron’s Density
Iron’s density is a fundamental property influenced by several factors that affect the arrangement and packing of atoms within its structure. These factors include temperature, purity, and alloying elements. Understanding the influence of these factors is crucial for optimizing iron’s properties and applications.
Temperature
Temperature significantly affects the density of iron. As temperature increases, the thermal energy of iron atoms increases, causing them to vibrate more vigorously. This increased vibration leads to a larger average distance between atoms, resulting in a decrease in density.
Conversely, when iron is cooled, the thermal energy decreases, and atoms pack more closely together, increasing the density.
Purity
The purity of iron also plays a role in determining its density. Impurities, such as carbon, silicon, and manganese, can alter the crystal structure of iron, affecting the arrangement of atoms. The presence of impurities can create defects or vacancies in the crystal lattice, which can lead to changes in density.
Generally, higher purity iron has a higher density compared to impure iron.
Alloying Elements
Alloying iron with other elements can significantly alter its density. When alloying elements are added to iron, they form new phases or compounds within the iron matrix. These new phases have different densities than pure iron, which can affect the overall density of the alloy.
For example, adding carbon to iron forms steel, which has a lower density than pure iron due to the presence of carbon atoms.
Applications of Iron’s Density
Iron’s high density of 7.86 g/cm³ plays a crucial role in its widespread applications across various industries. The density of iron directly influences its strength, durability, and resistance to deformation, making it a suitable material for a range of engineering and construction purposes.
Construction
In the construction industry, iron’s density contributes to its exceptional load-bearing capacity. Steel beams and girders, composed primarily of iron, are commonly used in the construction of bridges, skyscrapers, and other large-scale structures. The high density of iron ensures that these structures can withstand significant weight and forces without compromising their integrity.
Transportation
The transportation industry also benefits from iron’s high density. In the automotive sector, iron is used in the production of engine blocks, frames, and other critical components. The density of iron provides the necessary strength and durability to withstand the stresses and vibrations encountered during operation.
Similarly, in the shipbuilding industry, iron is used in the construction of hulls and decks, where its density contributes to the overall stability and buoyancy of vessels.
Manufacturing
Iron’s density also plays a vital role in the manufacturing sector. Iron castings are widely used in the production of machinery, tools, and equipment. The high density of iron provides the necessary strength and wear resistance for components subjected to high loads and abrasive conditions.
Additionally, iron’s density makes it suitable for use in the production of magnets, where its ability to retain magnetic properties is enhanced by its high density.
FAQ Overview
What is the significance of iron’s density?
Iron’s density plays a crucial role in determining its strength, durability, and resistance to deformation.
How does temperature affect iron’s density?
As temperature increases, the atoms within iron become more agitated, leading to a decrease in density.
What are the applications of iron’s high density?
Iron’s high density makes it suitable for use in construction, transportation, and manufacturing industries, where strength and durability are essential.