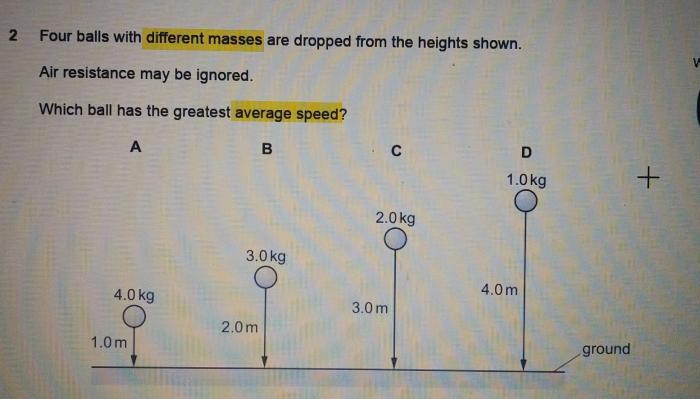
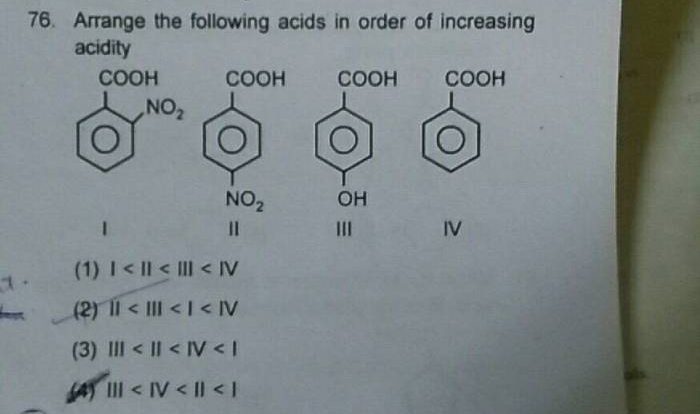
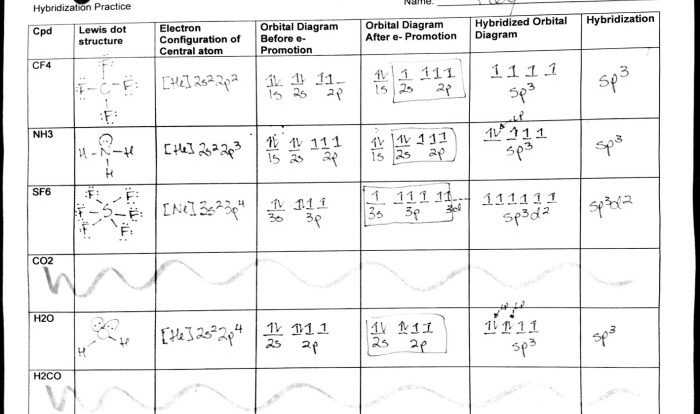
Embark on an enlightening journey with our practice drawing ionic bonds answer key, meticulously crafted to guide you through the intricacies of ionic bonding. Delve into the fundamental principles, explore real-world applications, and uncover the secrets of accurate ionic bond representation.
Our comprehensive guide provides step-by-step instructions, interactive exercises, and detailed explanations to empower you with a thorough understanding of ionic bonding.
Introduction: Practice Drawing Ionic Bonds Answer Key
Ionic bonding is a type of chemical bond formed between atoms or molecules with opposite electrical charges, resulting in the formation of an ionic compound. Ionic compounds are typically composed of a metal and a non-metal.
Ionic bonding occurs when one atom gives up one or more electrons to another atom. The atom that gives up electrons becomes positively charged, while the atom that receives electrons becomes negatively charged. The oppositely charged ions are attracted to each other by electrostatic forces, forming an ionic bond.
Ionic compounds are typically solids at room temperature and have high melting and boiling points. They are also good conductors of electricity when dissolved in water or melted.
Characteristics of Ionic Compounds, Practice drawing ionic bonds answer key
- Solid at room temperature
- High melting and boiling points
- Good conductors of electricity when dissolved in water or melted
- Formed between a metal and a non-metal
- Composed of positively and negatively charged ions
Examples of Ionic Compounds
- Sodium chloride (NaCl)
- Potassium chloride (KCl)
- Calcium fluoride (CaF2)
- Magnesium oxide (MgO)
- Iron(III) oxide (Fe2O3)
Practice Drawing Ionic Bonds
To draw an ionic bond, follow these steps:
- Identify the metal and non-metal atoms involved in the bond.
- Determine the number of electrons that the metal atom will give up and the number of electrons that the non-metal atom will receive.
- Write the chemical symbols for the metal and non-metal atoms, and indicate the charges of the ions by writing the number of electrons gained or lost as superscripts.
- Draw a line between the metal and non-metal ions to represent the ionic bond.
For example, to draw the ionic bond in sodium chloride (NaCl), we would follow these steps:
- Sodium is a metal, and chlorine is a non-metal.
- Sodium will give up one electron, and chlorine will receive one electron.
- The chemical symbols for sodium and chlorine are Na and Cl, respectively. The sodium ion has a charge of +1, and the chloride ion has a charge of
1.
- We can draw the ionic bond as follows:
Na ++ Cl –→ NaCl
Top FAQs
What is the significance of ionic bonding in biological systems?
Ionic bonding plays a crucial role in maintaining the structural integrity of biomolecules such as proteins and nucleic acids, facilitating cellular processes, and regulating nerve impulses.
How can I avoid common mistakes when drawing ionic bonds?
Pay attention to the charges of the ions, ensure proper electron transfer, and consider the size and electronegativity of the atoms involved.