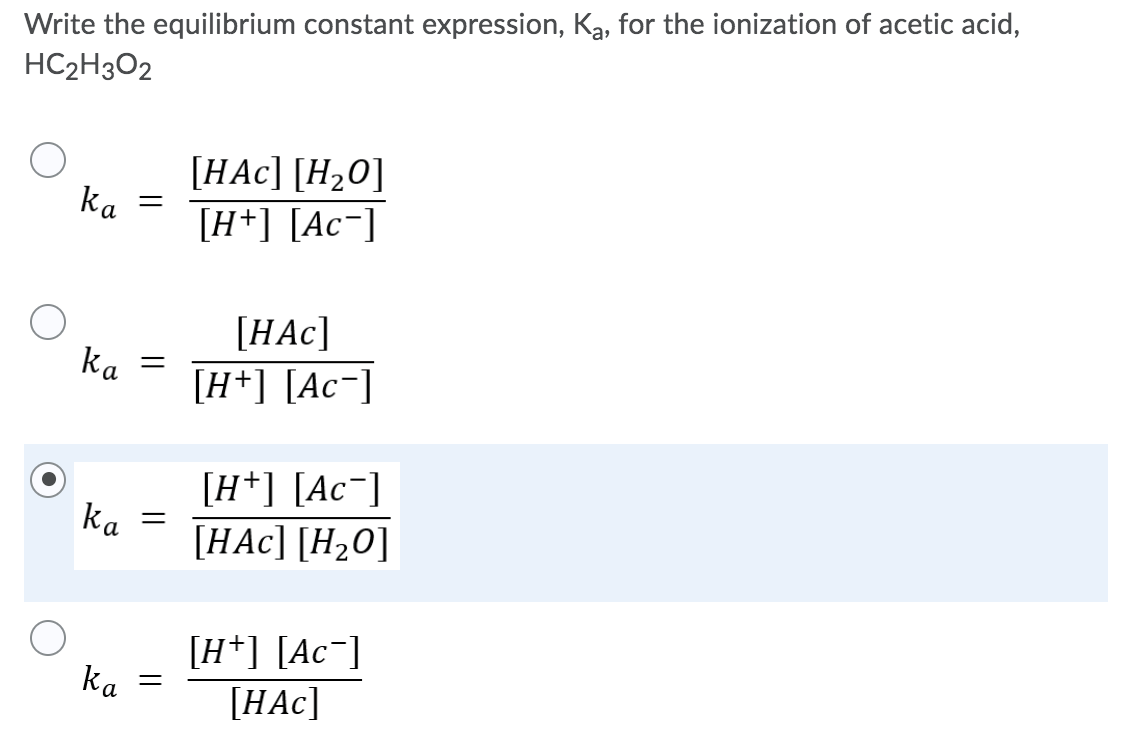The equilibrium for the acid ionization of hc2ho3 – The equilibrium for the acid ionization of HC2H3O2 is a fundamental concept in chemistry that plays a crucial role in understanding the behavior of acids and bases. This article delves into the intricacies of this equilibrium, exploring its expression, factors affecting it, and its applications in various fields.
The equilibrium constant, Ka, quantifies the extent of ionization and provides insights into the strength of the acid. Factors such as temperature, concentration, and ionic strength influence the equilibrium position, determining the relative amounts of ionized and unionized species.
Equilibrium for the Acid Ionization of HC2H3O2: The Equilibrium For The Acid Ionization Of Hc2ho3
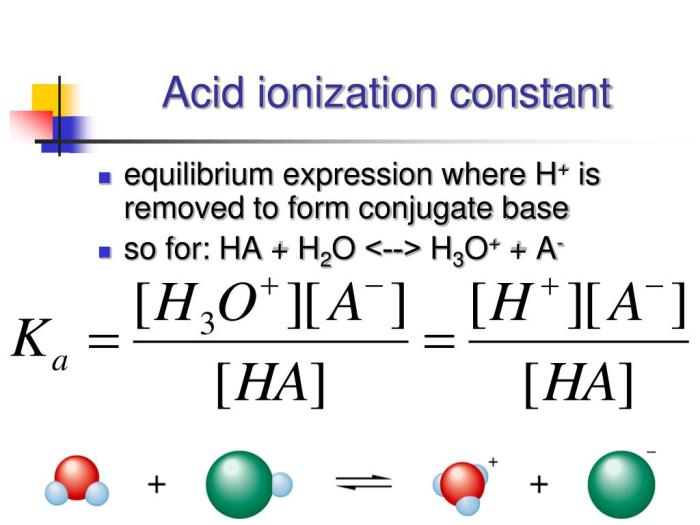
The acid ionization of HC2H3O2, commonly known as acetic acid, is a fundamental chemical equilibrium that plays a crucial role in various scientific disciplines. This equilibrium involves the dissociation of acetic acid into hydrogen ions (H+) and acetate ions (CH3COO-).
Equilibrium Expression and Constant
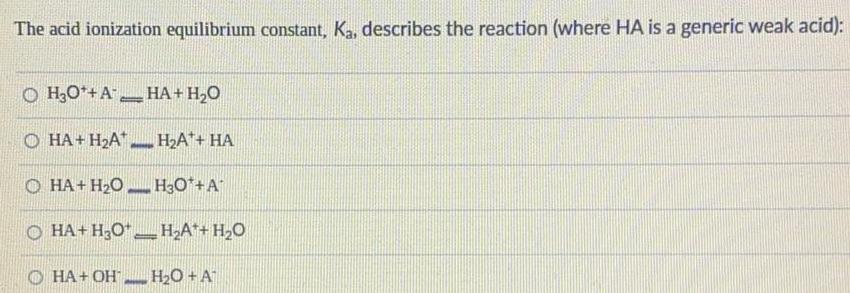
The equilibrium expression for the acid ionization of HC2H3O2 is given by:
Ka = [H+][CH3COO-] / [HC2H3O2]
where Ka is the equilibrium constant. The equilibrium constant is a measure of the extent to which the acid ionizes in water. A higher Ka value indicates a stronger acid, as it means that the acid ionizes more completely in water.
Factors Affecting Equilibrium
The equilibrium position of the acid ionization reaction is affected by several factors, including:
- Temperature:Increasing temperature favors the ionization of the acid, leading to a higher Ka value.
- Concentration:Increasing the initial concentration of HC2H3O2 shifts the equilibrium to the left, favoring the undissociated form.
- Ionic strength:Increasing the ionic strength of the solution decreases the Ka value, as the presence of other ions reduces the activity of the hydrogen ions.
pH Calculations
The pH of a solution containing HC2H3O2 can be calculated using the equilibrium expression and the Ka value. The pH is given by:
pH =
log[H+]
where [H+] is the molar concentration of hydrogen ions. By substituting the equilibrium expression for Ka into the pH equation, we get:
pH =
- log(Ka
- [HC2H3O2] / [CH3COO-])
Buffer Solutions
HC2H3O2 can be used as a component in buffer solutions. A buffer solution is a solution that resists changes in pH when small amounts of acid or base are added. Buffer solutions are important in biological systems, as they help to maintain a stable pH environment.
Titration Curves, The equilibrium for the acid ionization of hc2ho3
The titration curve for the titration of HC2H3O2 with a strong base shows a gradual increase in pH as the base is added. The equivalence point is reached when the moles of base added are equal to the moles of HC2H3O2 initially present.
At the equivalence point, the pH is equal to pKa, which is the negative logarithm of the Ka value.
Applications
The equilibrium for the acid ionization of HC2H3O2 has important applications in various fields, including:
- Biological systems:Acetic acid is a weak acid that is produced as a byproduct of cellular metabolism. The equilibrium between HC2H3O2 and its ions plays a role in maintaining the pH of biological fluids.
- Industrial processes:Acetic acid is used in the production of a variety of chemicals, including vinegar, plastics, and pharmaceuticals.
- Environmental chemistry:Acetic acid is a component of atmospheric aerosols and can contribute to acid rain.
FAQ Summary
What is the equilibrium expression for the acid ionization of HC2H3O2?
The equilibrium expression is: Ka = [H+][C2H3O2-] / [HC2H3O2]
How does temperature affect the equilibrium position?
Increasing temperature generally shifts the equilibrium towards ionization, resulting in a higher concentration of H+ and C2H3O2- ions.
What is the role of HC2H3O2 in buffer solutions?
HC2H3O2 can act as a buffer component by resisting changes in pH when small amounts of acid or base are added.