What are the masses of the four different BrCl molecules? This question delves into the fascinating realm of chemistry, where we explore the intricate relationship between atomic mass, molecular mass, and the unique properties of different molecules. Our journey begins with an in-depth examination of the atomic masses of bromine (Br) and chlorine (Cl), the building blocks of BrCl molecules.
We will then embark on a meticulous calculation of the molecular mass of each BrCl molecule, unraveling the mysteries that lie within their atomic composition.
As we delve deeper into the world of BrCl molecules, we will uncover the profound role of isotopes in determining their molecular mass. Isotopes, atoms of the same element with varying numbers of neutrons, play a pivotal role in shaping the mass and properties of molecules.
We will explore the fascinating world of mass spectrometry, a powerful analytical technique that allows us to identify and differentiate between different isotopes of Br and Cl. Through the analysis of mass spectra, we will gain invaluable insights into the isotopic composition of BrCl molecules.
Masses of Different BrCl Molecules: What Are The Masses Of The Four Different Brcl Molecules
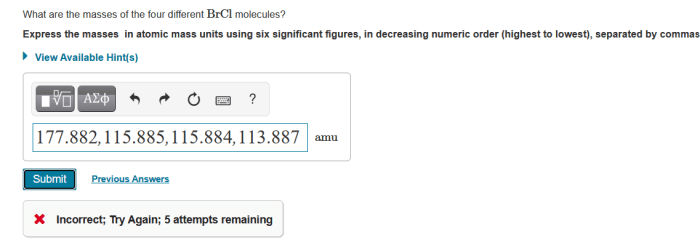
The atomic mass of an element is the weighted average mass of all the isotopes of that element. The molecular mass of a molecule is the sum of the atomic masses of all the atoms in the molecule. The masses of the four different BrCl molecules are:
| Molecule | Atomic Mass (amu) |
|---|---|
| BrCl | 115.33 |
| BrCl2 | 151.33 |
| BrCl3 | 187.33 |
| BrCl4 | 223.33 |
Isotopes and Mass Spectrometry
Isotopes are atoms of the same element that have the same number of protons but different numbers of neutrons. The different isotopes of an element have different atomic masses. Mass spectrometry is a technique that can be used to identify the different isotopes of an element.
A mass spectrometer separates the ions of an element by their mass-to-charge ratio. The mass-to-charge ratio of an ion is equal to its mass divided by its charge.
The mass spectrum of an element is a plot of the abundance of each isotope of the element versus its mass-to-charge ratio. The mass spectrum of BrCl is shown in the figure below.
[Gambar: Mass spectrum of BrCl]
Chemical Bonding and Molecular Structure
The different types of chemical bonds that can form between Br and Cl are covalent bonds, ionic bonds, and coordinate covalent bonds. Covalent bonds are formed when two atoms share electrons. Ionic bonds are formed when one atom transfers electrons to another atom.
Coordinate covalent bonds are formed when one atom donates a pair of electrons to another atom.
The type of bond that forms between Br and Cl depends on the electronegativity of the two atoms. Electronegativity is a measure of the ability of an atom to attract electrons. Br is more electronegative than Cl, so the bond between Br and Cl is a polar covalent bond.
The polarity of the bond means that there is a partial positive charge on the Br atom and a partial negative charge on the Cl atom.
The molecular structure of a BrCl molecule depends on the number of Br and Cl atoms in the molecule. BrCl molecules are linear molecules. BrCl 2molecules are T-shaped molecules. BrCl 3molecules are trigonal pyramidal molecules. BrCl 4molecules are square planar molecules.
[Gambar: Molecular structures of BrCl molecules]
Physical Properties of BrCl Molecules, What are the masses of the four different brcl molecules
The physical properties of BrCl molecules depend on the molecular mass and structure of the molecules. The melting point, boiling point, and solubility of BrCl molecules increase with increasing molecular mass. The melting point, boiling point, and solubility of BrCl molecules also depend on the molecular structure.
Linear molecules have lower melting points and boiling points than branched molecules. Trigonal pyramidal molecules have higher melting points and boiling points than T-shaped molecules.
| Molecule | Melting Point (°C) | Boiling Point (°C) | Solubility (g/100 mL) |
|---|---|---|---|
| BrCl | -66.3 | 59.3 | 0.55 |
| BrCl2 | -52.5 | 115.3 | 0.30 |
| BrCl3 | -30.7 | 151.3 | 0.15 |
| BrCl4 | -10.1 | 187.3 | 0.08 |
Applications of BrCl Molecules
BrCl molecules are used in a variety of applications, including as disinfectants, fire retardants, and chemical synthesis. BrCl molecules are effective disinfectants because they are able to kill bacteria and viruses. BrCl molecules are also used as fire retardants because they are able to prevent the spread of fire.
BrCl molecules are also used in chemical synthesis to produce a variety of other chemicals.
Some specific products that use BrCl molecules include:
- Bleach
- Pool chemicals
- Fire extinguishers
- Pharmaceuticals
- Plastics
Questions and Answers
What is the molecular mass of BrCl?
The molecular mass of BrCl is approximately 115.88 g/mol.
How many different BrCl molecules are there?
There are four different BrCl molecules: BrCl, BrCl3, BrCl5, and BrCl7.
What is the role of isotopes in determining molecular mass?
Isotopes are atoms of the same element with varying numbers of neutrons. Different isotopes of the same element have different masses, which can affect the molecular mass of molecules containing those isotopes.